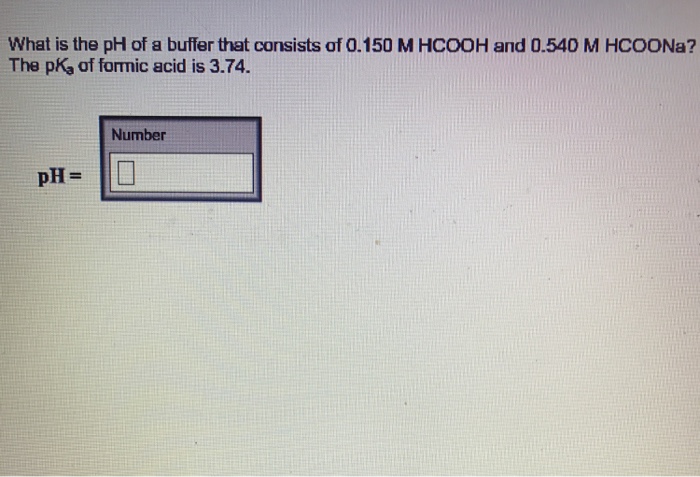
SOLVED: A formic acid buffer containing 0.50 M HCOOH and 0.50 M HCOONa has a pH of 3.77. What will the pH be after 0.010 mol of NaOH has been added to
![SOLVED: Question 10 (1 point) A 1.00 L solution of a HCOOH/HCOONa buffer has pH 3.60,and [HCOONa] = 0.68M. After 50.0 mL of 1.00 M HCI are added, what is the new SOLVED: Question 10 (1 point) A 1.00 L solution of a HCOOH/HCOONa buffer has pH 3.60,and [HCOONa] = 0.68M. After 50.0 mL of 1.00 M HCI are added, what is the new](https://cdn.numerade.com/ask_previews/5d3f1114-374a-4861-919c-a3549a935144_large.jpg)
SOLVED: Question 10 (1 point) A 1.00 L solution of a HCOOH/HCOONa buffer has pH 3.60,and [HCOONa] = 0.68M. After 50.0 mL of 1.00 M HCI are added, what is the new

How many moles of HCOONa must be added to 1 L of 0.1 M HCOOH to prepare a buffer solution with a pH of 3.4 ?(Given: Ka for HCOOH = 2 × 10^-4 )
what volume of 0.1M HCOONa should be added to 50ml of 0.05M HCOOH to produce a buffer of pH=4 if p^Ka=3.7

The pH values 0.1 M solution of HCOONa (I), HCOOH (II),CH(3)COONH(4) (III), NaOH (IV) HCl (V), will be in the order :

How many moles of HCOONa must be added to 1 L of 0.1 M HCOOH to prepare a buffer solution with a pH of 3.4 ?(Given: Ka for HCOOH = 2 × 10^-4 )

SOLVED:Use information from Appendix D to calculate the pH of (a) a solution that is 0.250M in sodium formate (HCOONa) and 0.100M in formic acid (HCOOH), (b) a solution that is 0.510M

SOLVED: Calculate the pH of a 0.500 L buffer solution composed of 0.700 M formic acid (HCOOH, Ka = 1.77 x 10¯4) and 0.500 M sodium formate (HCOONa).
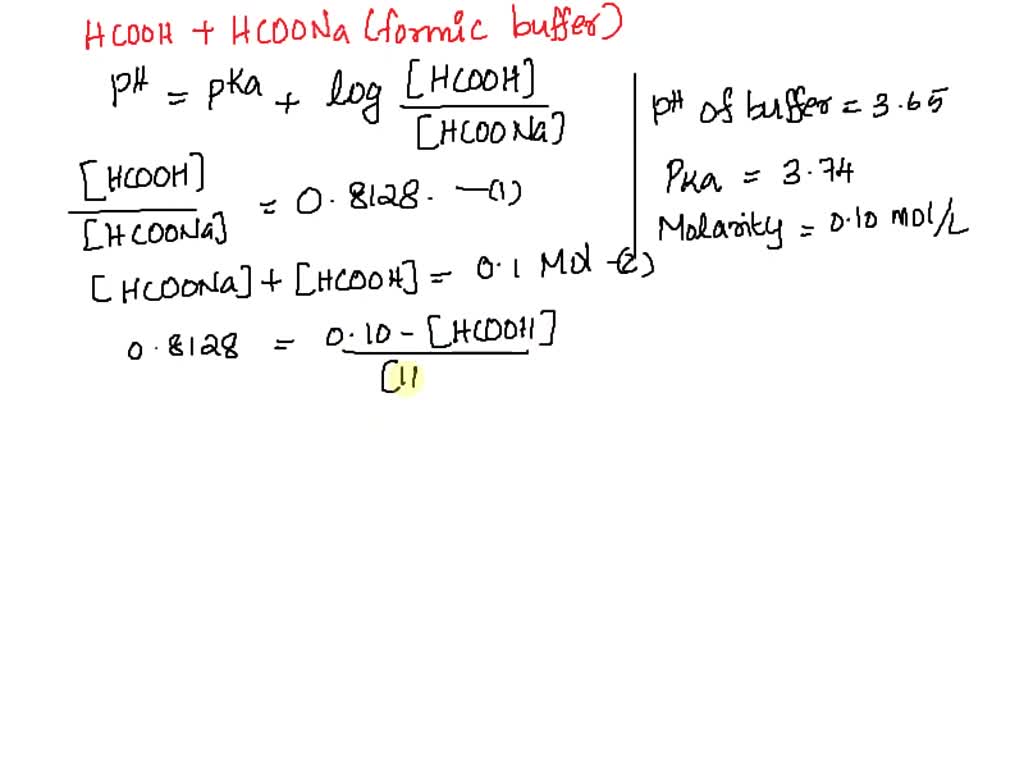
SOLVED: You have to prepare a pH 3.60 buffer, and you have the following 0.10M solutions available: HCOOH, CH3COOH, H3PO4, HCOONa, CH3COONa, and NaH2PO4. How many milliliters of HCOOH and HCOONa would

What volume of `0.1 M HCOONa` solution should be added to `50ml` of `0.05 M` formic acid to produce - YouTube
![SOLVED: A 1.00 L solution of HCOOH/HCOONa buffer has pH 3.60, and [HCOONa] = 0.68 M. After 50.0 mL of 1.00 M HCl are added, what is the new pH? data Ka (HCOOH)= 1.8X10^-4 SOLVED: A 1.00 L solution of HCOOH/HCOONa buffer has pH 3.60, and [HCOONa] = 0.68 M. After 50.0 mL of 1.00 M HCl are added, what is the new pH? data Ka (HCOOH)= 1.8X10^-4](https://cdn.numerade.com/ask_previews/a411b292-c627-429c-b44d-c4e267c7c0bc_large.jpg)
SOLVED: A 1.00 L solution of HCOOH/HCOONa buffer has pH 3.60, and [HCOONa] = 0.68 M. After 50.0 mL of 1.00 M HCl are added, what is the new pH? data Ka (HCOOH)= 1.8X10^-4

How many moles of HCOONa must be added to 1 L of 0.1 M HCOOH to prepare a buffer solution with a pH of 3.4 ?(Given: Ka for HCOOH = 2 × 10^-4 )




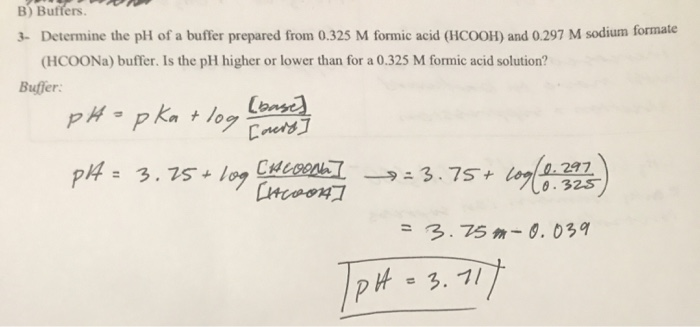

![Solved Find the pH of a buffer in which [HCOOH] = 1.24 M and | Chegg.com Solved Find the pH of a buffer in which [HCOOH] = 1.24 M and | Chegg.com](https://d2vlcm61l7u1fs.cloudfront.net/media%2F57e%2F57e106aa-e8ff-4851-b8b2-1e555776ca5e%2Fimage)