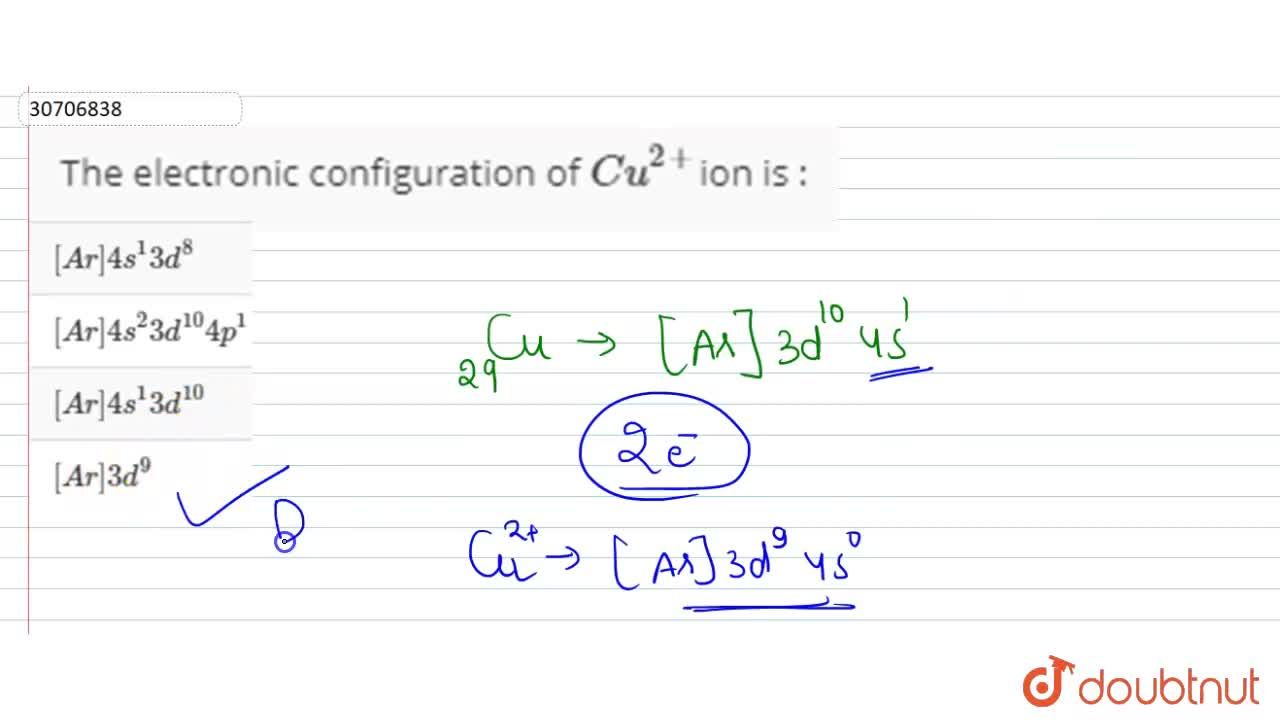
The electrode potentials for: Cu^2 + (aq) + e^ - → Cu^ + (aq) Cu^ + (aq) + e^ - → Cu (s) are + 0.15 V and 0.50 V respectively. The value of E^0 cu^2/Cu will be :

When `Cu^(2+)` ion is treated with KI, a white precipitate is formed. Explain the reaction with - YouTube

METTLER TOLEDO™ Ion Selective Electrodes (ISE) PerfectION Combined ISE; Measures Cu+2 ions; 1.2m cable, BNC METTLER TOLEDO™ Ion Selective Electrodes (ISE) | Fisher Scientific
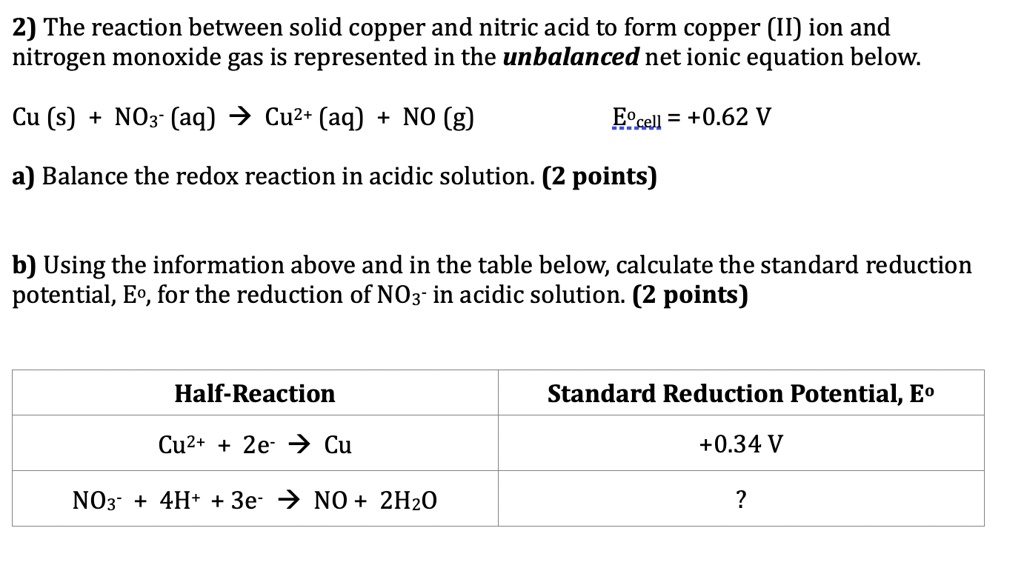
SOLVED: 2) The reaction between solid copper and nitric acid to form copper (II) ion and nitrogen monoxide gas is represented in the unbalanced net ionic equation below: Cu (s) NOz" (aq)

In this example, we'll show you how to write the formula for an ionic compound with a multivalent metal. - ppt download

Given:(i) Cu^2 + + 2e^-→ Cu, E^∘ = 0.337 V (ii) Cu^2 + + e^-→ Cu^+, E^∘ = 0.153 V Electrode potential, E^∘ for the reaction, Cu^+ + e^-→ Cu , will be

Consider the reaction below: \\ Zn (s) + Cu2+ (aq) Zn2+(aq) + Cu (s) \\ a) Write two half reactions with their respective E0 value. \\ b) Draw a diagram of the

Cu2+ + 2e– → Cu E° = + 0.34 VAg+ + 1e– → Ag E° = + 0.80 V(i) Construct a galvanic cell using the above data.(ii) For what concentration of Ag+ ions
22. The electrode potentials for Cu2+ +e —> Cu+ and Cu+ +e —>Cu are +0.15V and +0.50V respectively.The value of E(Cu2+/Cu ) will be 1.0.150V 2.0.500V 3.0.325V 4.0.650V

SOLVED: How many protons, neutrons and electrons are there in a Cu2+ ion with a mass number of 63 ? 63, 32, 29 29, 34, 27 34, 29, 27 29, 34, 31 63, 29, 31