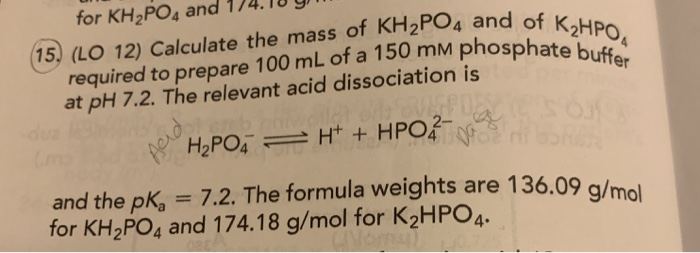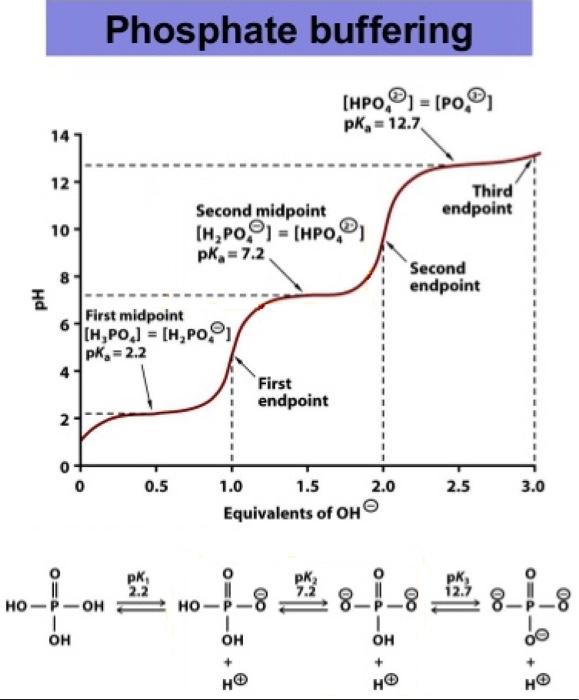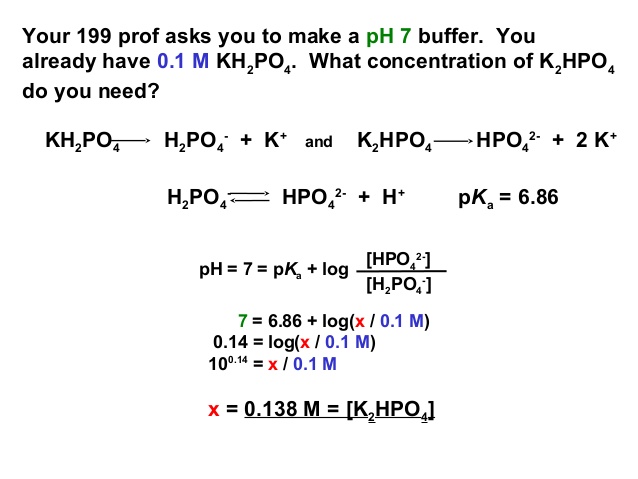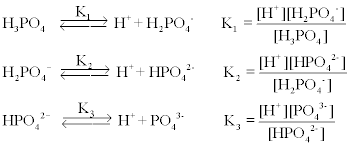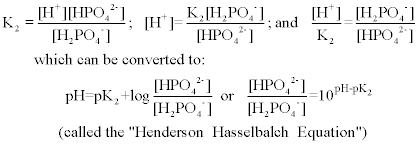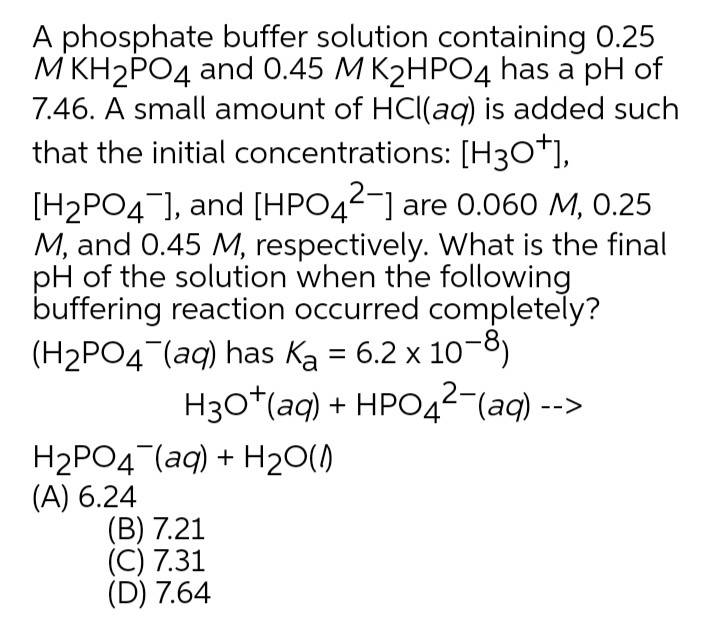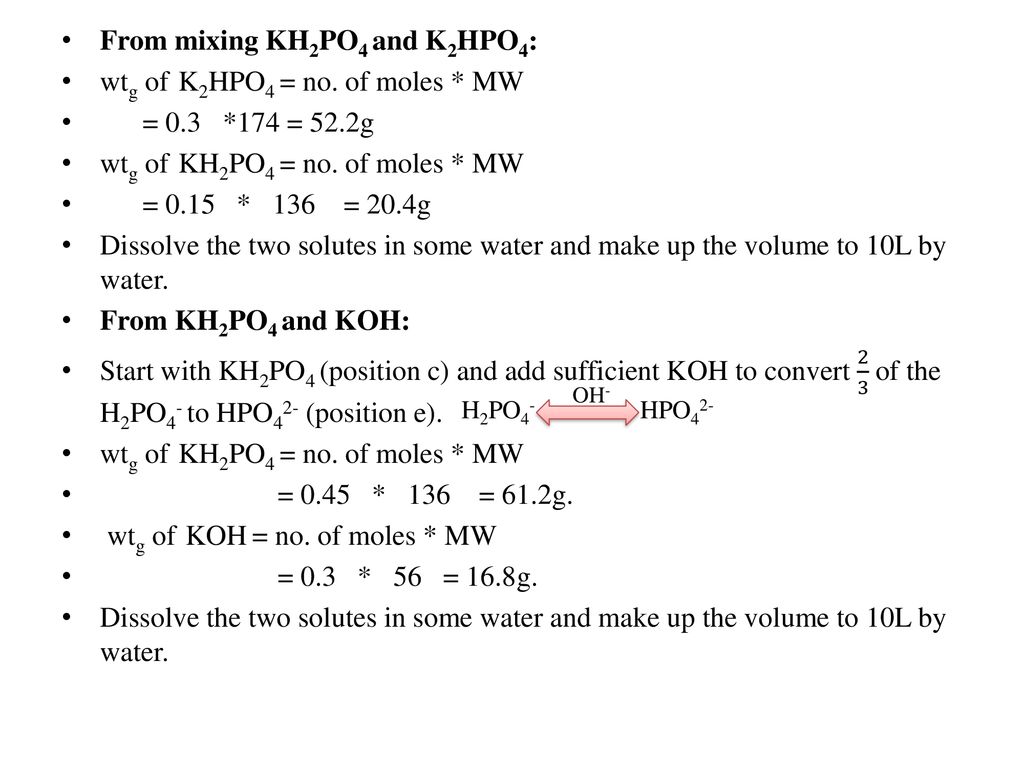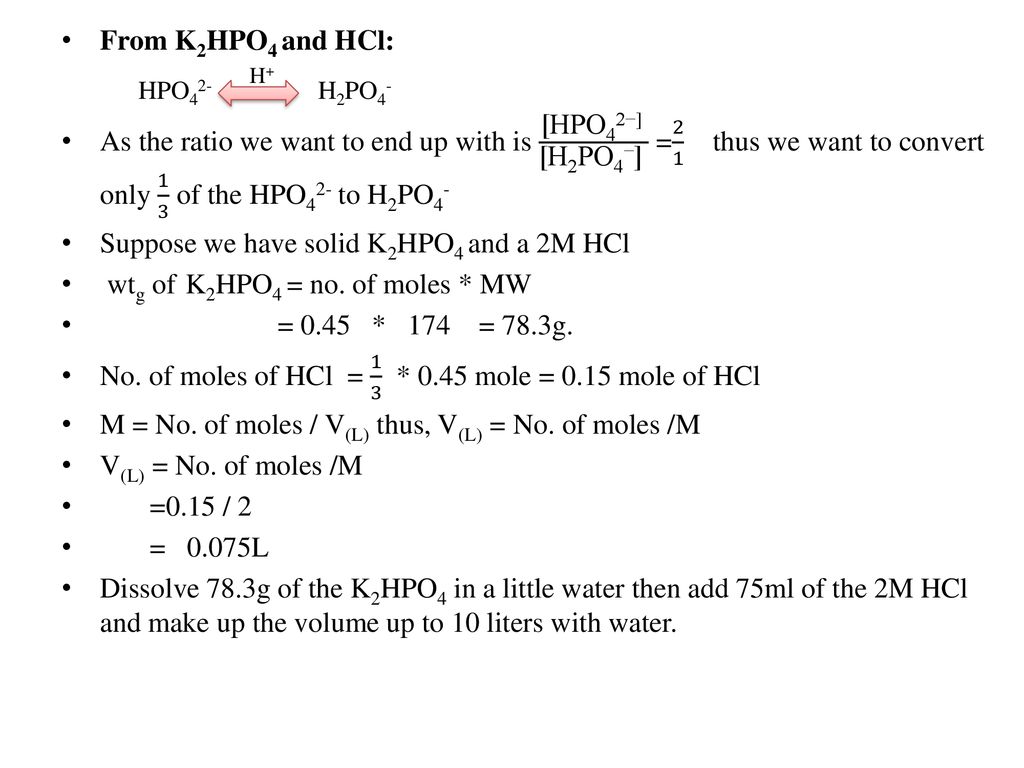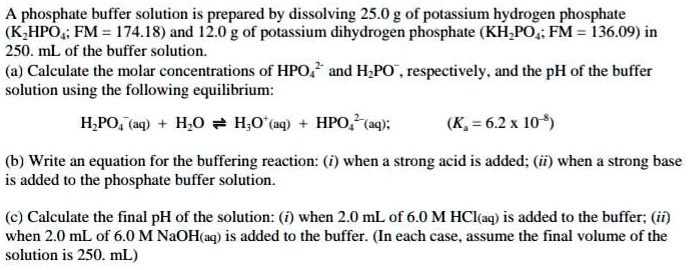
SOLVED: A phosphate buffer solution is prepared by dissolving 25.0 g of potassium hydrogen phosphate (K,HPO ; FM = 174.18) and 12.0 g of potassium dihydrogen phosphate (KH,PO : FM = 136.09)
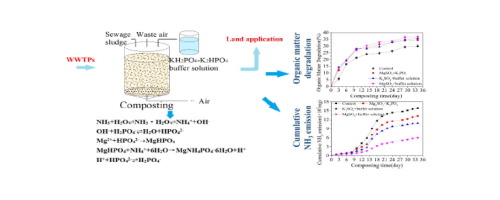
Nitrogen loss reduction by adding KH2PO4-K2HPO4 buffer solution during composting of sewage sludge,Bioresource Technology - X-MOL

Control of D value by mixing different amounts of K2HPO4/KH2PO4 salts.... | Download Scientific Diagram

OneClass: Write the chemical reaction for:KH2PO4/K2HPO4 buffer solution + NaOH (aq)andWrite the chemi...

SOLVED: In order to create a buffer zone at physiological condition (neutral pH) the potassium phosphate buffer is to be made of: K3PO4 and KzHPO4 KzHPO4 and KH2PO4 KHzPO4 and HzPO4 KzHPO4

Nitrogen loss reduction by adding KH2PO4-K2HPO4 buffer solution during composting of sewage sludge - ScienceDirect
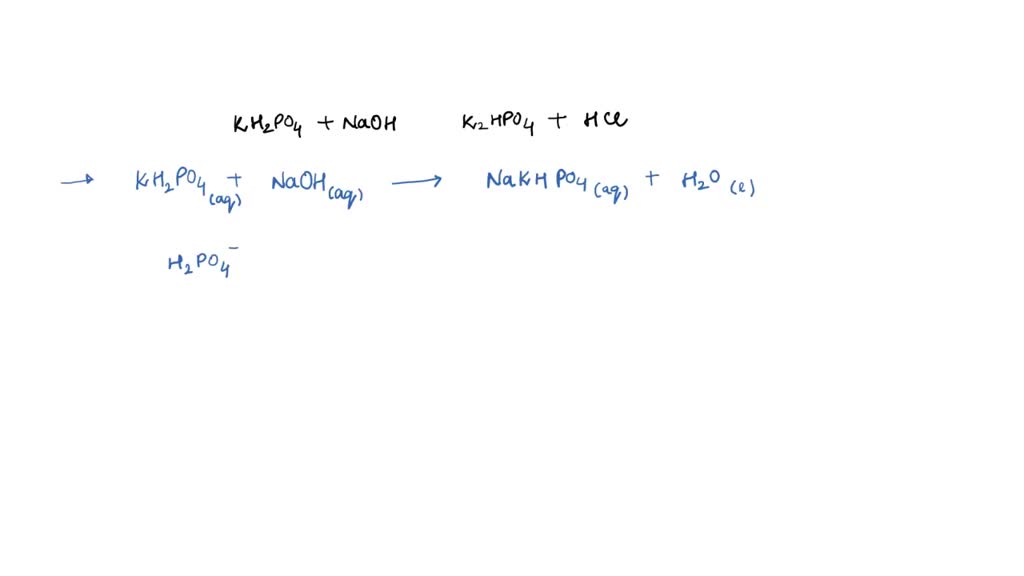
SOLVED: Write the equation and the reaction of the buffer solution KH2PO4 / K2HPO4 when NaOH and HCl is added

Concentrations of potassium phosphate buffer and rham- nolipid investigated | Download Scientific Diagram