
Use of the Data Booklet is relevant to this question.Which diagram correctly shows the electronegativity of the elements Na, Mg, Al and Si plottedagainst their first ionisation energies?

In modern periodic table, arrange the third row elements Na, Mg, AI and Si in the increasing order of their atomic size.

Na, Mg and Al are the elements having one, two and 3 valence electrons respectively Which of these elements - Science - Periodic Classification of Elements - 14139331 | Meritnation.com

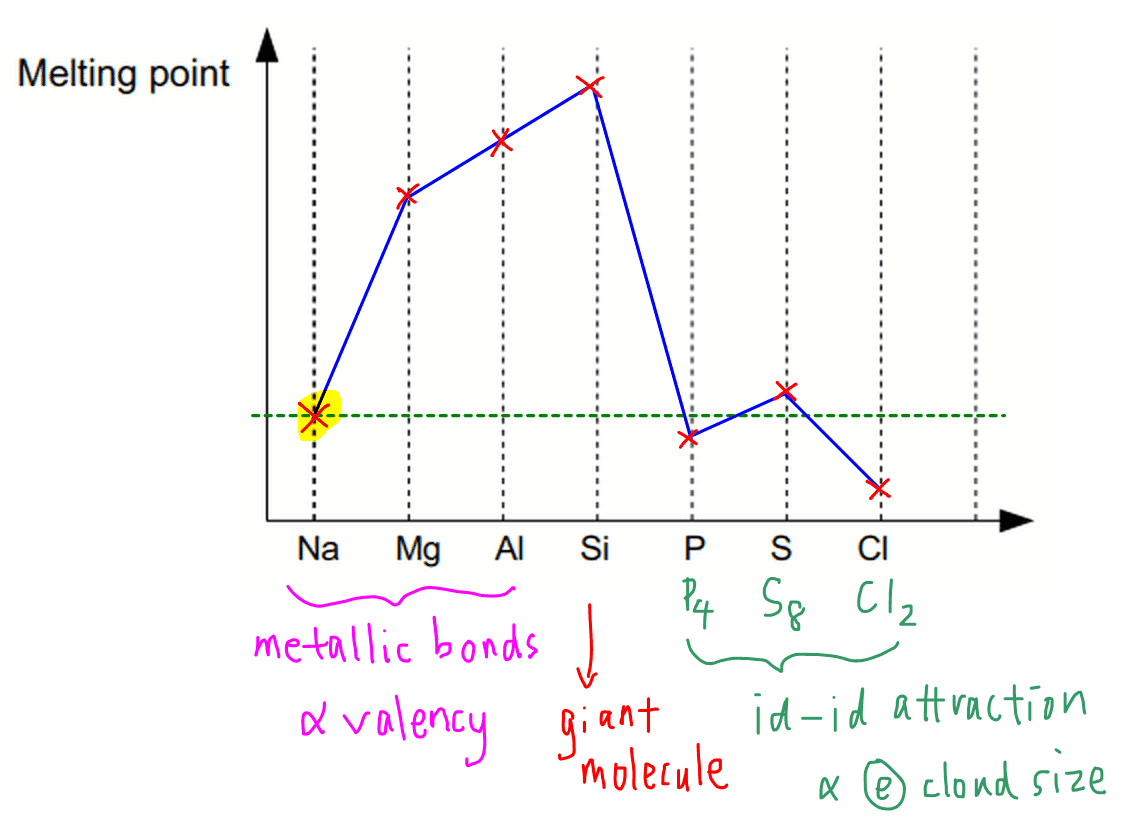





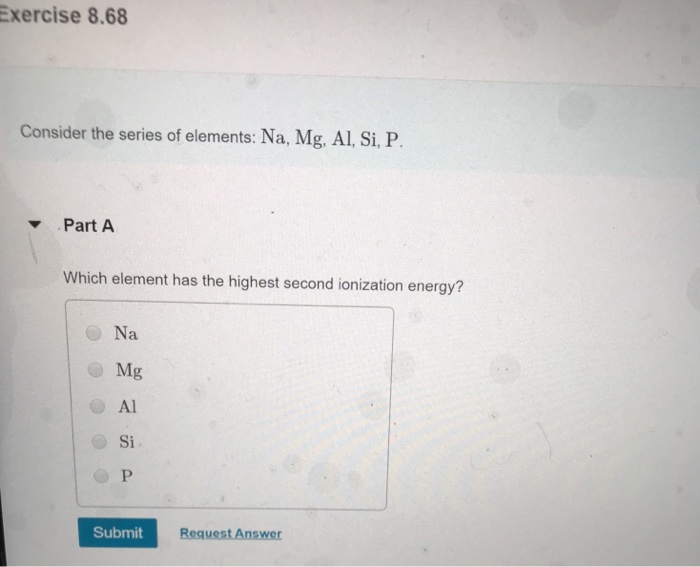

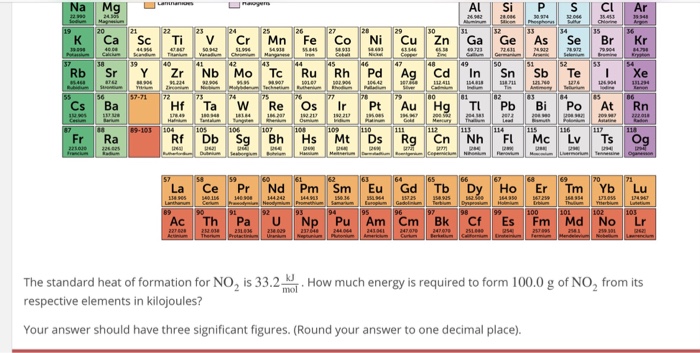




![The amount of Si, Al, Ca, K, Na, Mg and Fe in examined samples [%]... | Download Table The amount of Si, Al, Ca, K, Na, Mg and Fe in examined samples [%]... | Download Table](https://www.researchgate.net/publication/315174935/figure/tbl1/AS:651113229463552@1532248899349/The-amount-of-Si-Al-Ca-K-Na-Mg-and-Fe-in-examined-samples-Power-Plant-1_Q320.jpg)
