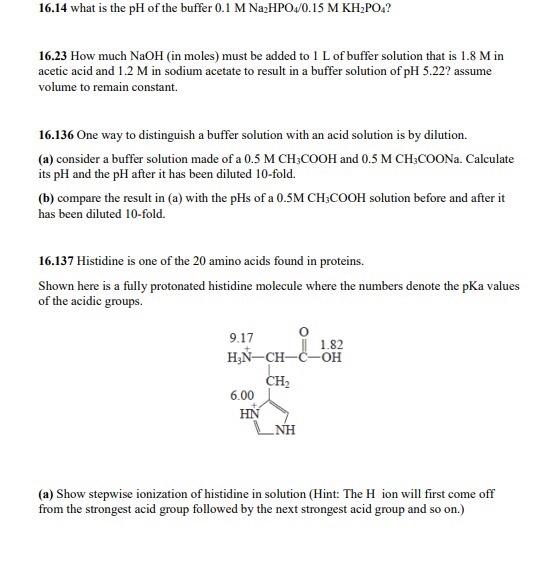
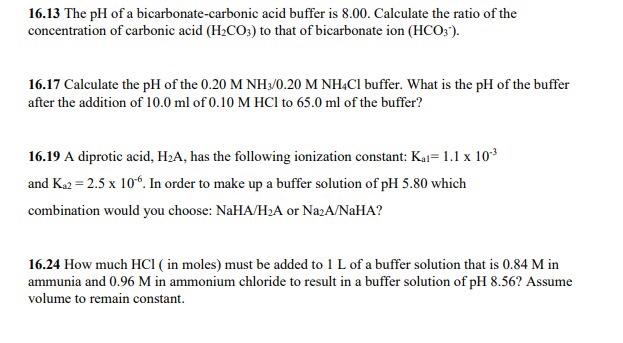
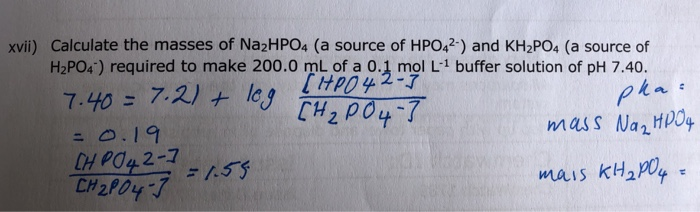Gel filtration chromatography for purified β-lactamase enzyme from A.... | Download Scientific Diagram

SOLVED: A buffer solution is 0.362 M in H3PO4 and 0.219 M in KH2PO4 . If Ka1 for H3PO4 is 7.5 x 10^-3 , what is the pH of this buffer solution? pH=
![Buy PBST [10X]; Phosphate Buffered Saline with Tween-20 Solution; 80mM Na2HPO4, 1.5M NaCl, 20mM KH2PO4, 30mM KCl, 0.5% Tween-20, pH 7.4-1 L -from Cepham Life Sciences Online at desertcartBulgaria Buy PBST [10X]; Phosphate Buffered Saline with Tween-20 Solution; 80mM Na2HPO4, 1.5M NaCl, 20mM KH2PO4, 30mM KCl, 0.5% Tween-20, pH 7.4-1 L -from Cepham Life Sciences Online at desertcartBulgaria](https://m.media-amazon.com/images/W/WEBP_402378-T1/images/I/31Hl1HwULnL.jpg)
Buy PBST [10X]; Phosphate Buffered Saline with Tween-20 Solution; 80mM Na2HPO4, 1.5M NaCl, 20mM KH2PO4, 30mM KCl, 0.5% Tween-20, pH 7.4-1 L -from Cepham Life Sciences Online at desertcartBulgaria

FT-IR spectral profiles of NaH2PO4, Na2HPO4, and KH2PO4. KH2PO4, red... | Download Scientific Diagram

OneClass: Part A: Suppose you wanted to make a buffer of exactly pH 7.00 using KH2PO4 and Na2HPO4 . I...

SOLVED: 8. To make up a solution of phosphate buffered saline (PBS), you need 15 mM Na2HPO4 (anhydrous) (FW: 141.96 g/mol), 0.25 M NaCl (FW: 58.44 g/mol), and 2 mM KH2PO4 (FW:
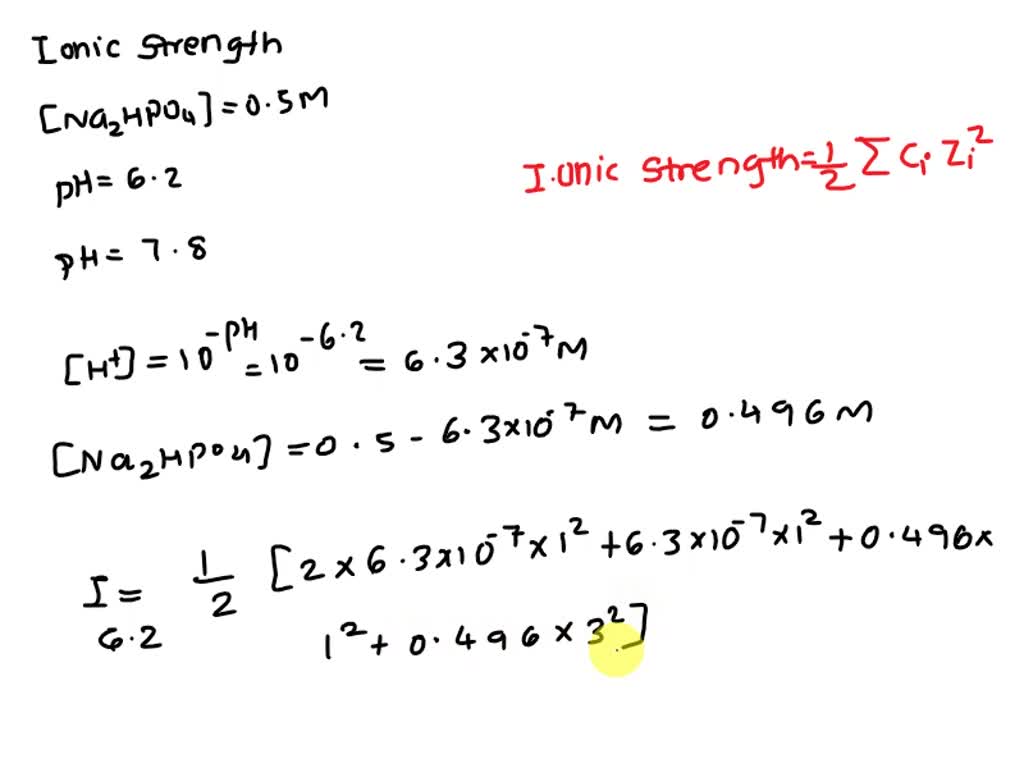
SOLVED: A buffer solution was prepared by mixing 0.1M K2HPO2 and 0.1M KH2PO4 (pH 6.64) - calculate the ionic strength (I) of the standard phosphate buffer solution
![PBS [1X] [137mM NaCl, 2.7mM KCl, 10mM KH2PO4, 1.8mM NaH2PO4, pH 7.4] - Cepham Life Sciences Research Products PBS [1X] [137mM NaCl, 2.7mM KCl, 10mM KH2PO4, 1.8mM NaH2PO4, pH 7.4] - Cepham Life Sciences Research Products](https://www.cephamls.com/wp-content/uploads/2019/02/PBS-1X-1L.png)
PBS [1X] [137mM NaCl, 2.7mM KCl, 10mM KH2PO4, 1.8mM NaH2PO4, pH 7.4] - Cepham Life Sciences Research Products
![SOLVED: What is the pH of the buffer system made up of 0.10 M Na2HPO4/0.15 M KH2PO4? [ H2PO4- dissociation constant Ka = 6.2x10-8] 8.6 1.5 10.0 None of these SOLVED: What is the pH of the buffer system made up of 0.10 M Na2HPO4/0.15 M KH2PO4? [ H2PO4- dissociation constant Ka = 6.2x10-8] 8.6 1.5 10.0 None of these](https://cdn.numerade.com/ask_previews/a5a00885-2780-4697-a3a6-6966ddf39b81_large.jpg)
SOLVED: What is the pH of the buffer system made up of 0.10 M Na2HPO4/0.15 M KH2PO4? [ H2PO4- dissociation constant Ka = 6.2x10-8] 8.6 1.5 10.0 None of these
![Sodium Phosphate Dibasic (Na2HPO4, 500g) [CN03-500G] - $20.00 : Bioland Scientific, for Your Research Needs Sodium Phosphate Dibasic (Na2HPO4, 500g) [CN03-500G] - $20.00 : Bioland Scientific, for Your Research Needs](https://www.bioland-sci.com/images/Na2HPO4s%20500G.jpg)
Sodium Phosphate Dibasic (Na2HPO4, 500g) [CN03-500G] - $20.00 : Bioland Scientific, for Your Research Needs

Calculate the pH of a buffer solution obtained by dissolving 25.0 g of KH2PO4(s) and 38.0 g of Na2HPO4(s) in water and then diluting to 1.00 L. | Homework.Study.com
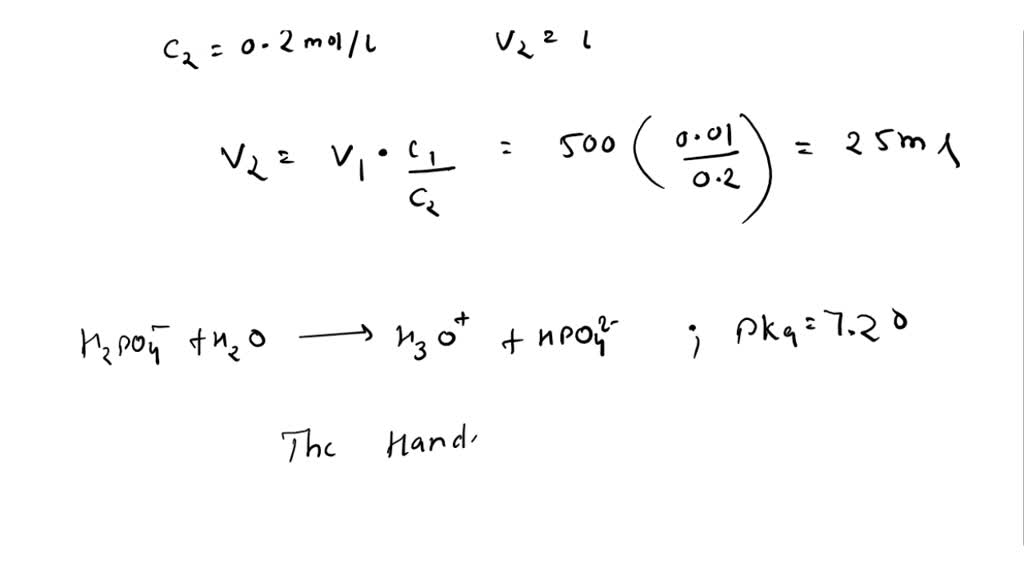
SOLVED: Suppose you wanted to make a buffer of exactly pH 7.00 using KH2PO4 and Na2HPO4. If the final solution was 0.1 M in KH2PO4, what concentration of Na2HPO4 would you need?
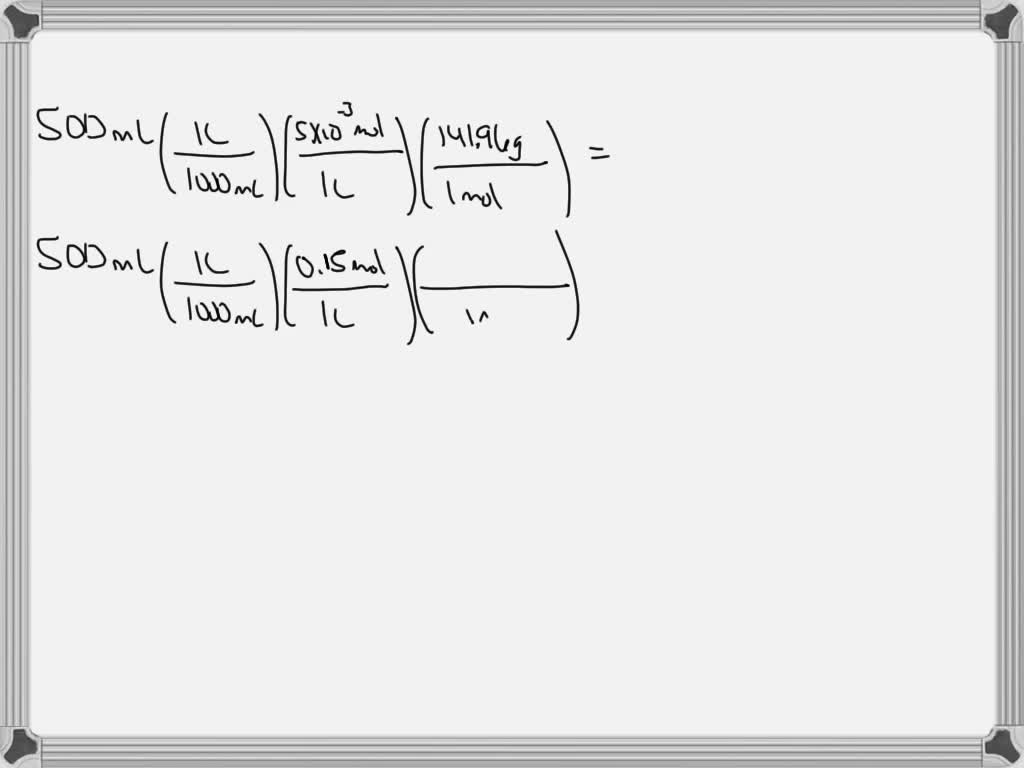
SOLVED: My answers were incorrect To make up a solution of phosphate buffered saline (PBS), you need 5mM Na2HPO4 (anhydrous) (FW: 141.96), 0.15 M NaCl (FW: 58.44), and 1 mM KH2PO4 (FW: