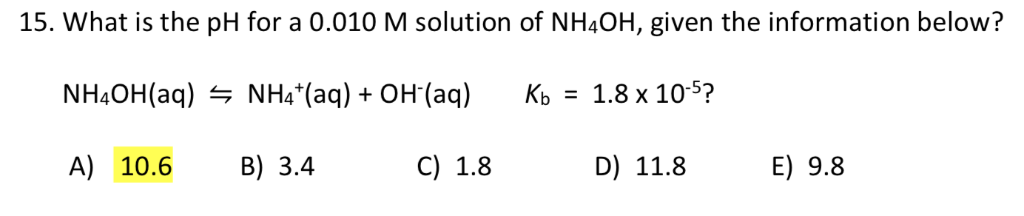
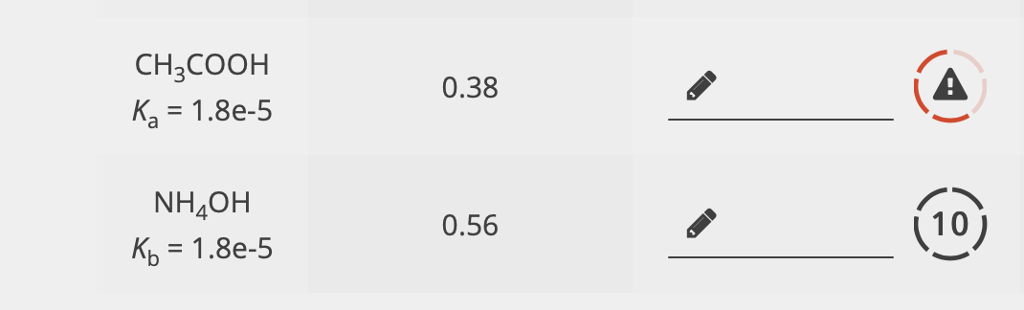
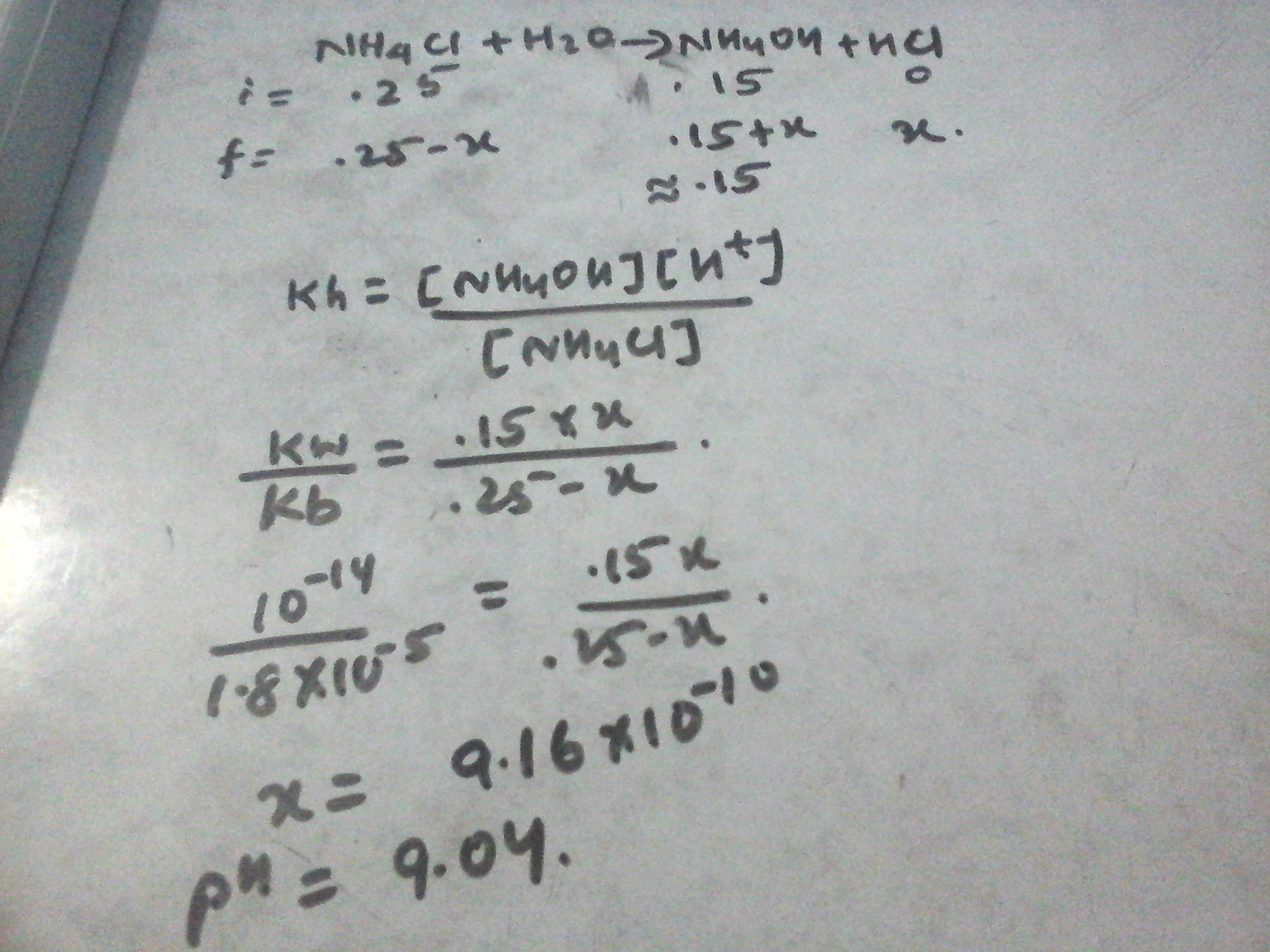SOLVED: What is the pH of a 0.15 M NH4OH solution with a Kb of 1.78x10^-5? a) 12.09 b) 11.93 c) 10.85 d) 11.21
Welcome to Chem Zipper.com......: Calculate the emf of the cell: Pt H2(1atm)ICH3COOH(0.1M) II NH4OH(0.01M)IH2(1 atm)Pt and Ka for CH3COOH= 1.8x10^-5 and Kb for NH4OH = 1.8x10^-5

Ka for CH3COOH is 1.8xx10^(-5) and Kb for NH4OH is 1.8xx10^(-5) The pH of ammonium acetate will be :

Sebanyak 0,1 mol NH4OH (Kb = 10-5) di campurkan dengan 0,05 mol NH4Cl hingga volume 1 liter - Mas Dayat
Calculate the percentage dissociation of 0.01 M NH4 OH solution. The Kb for NH4OH is 1.75 × 10^(-5). - Sarthaks eConnect | Largest Online Education Community
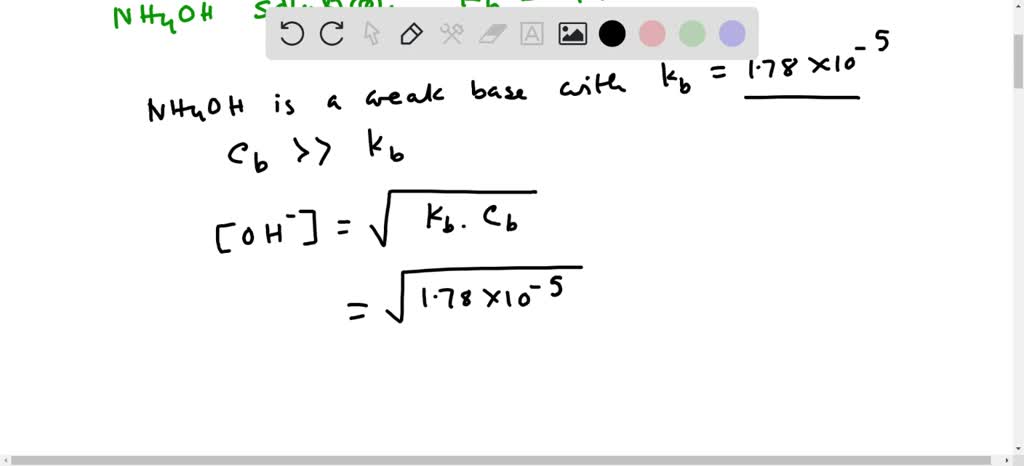
SOLVED: Answer the following equation:Ammonium Hydroxide, NH4OH is a weak base. Calculate the pH of 0.60 M solution of ammonium hydroxide. Kb= 1.78 x 10^-5
Welcome to Chem Zipper.com......: What will be the pH of the buffer solution containing 0.15 moles of NH4OH and 0.25 moles of NH4Cl Kb for NH4OH is 1.8x10^-5.