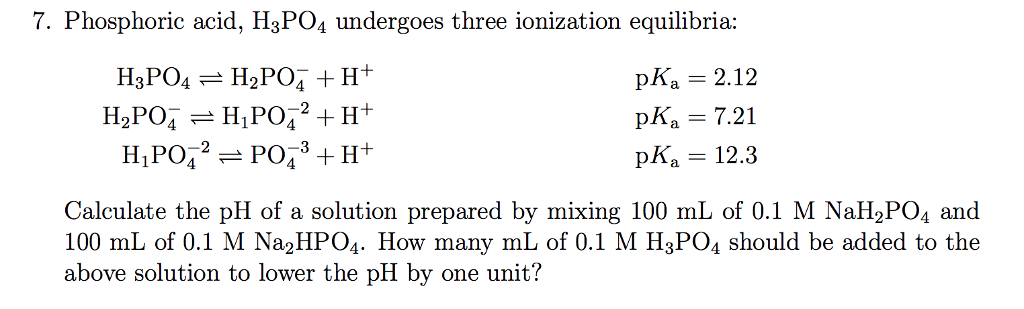
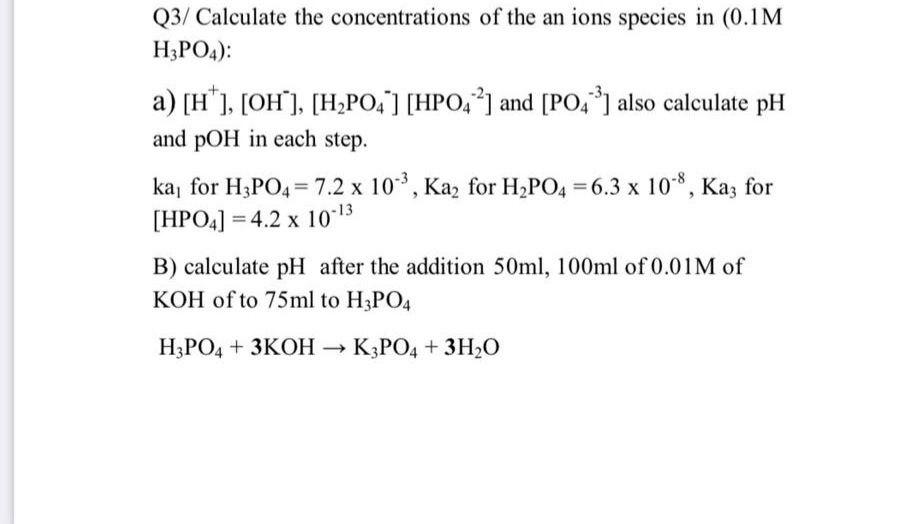
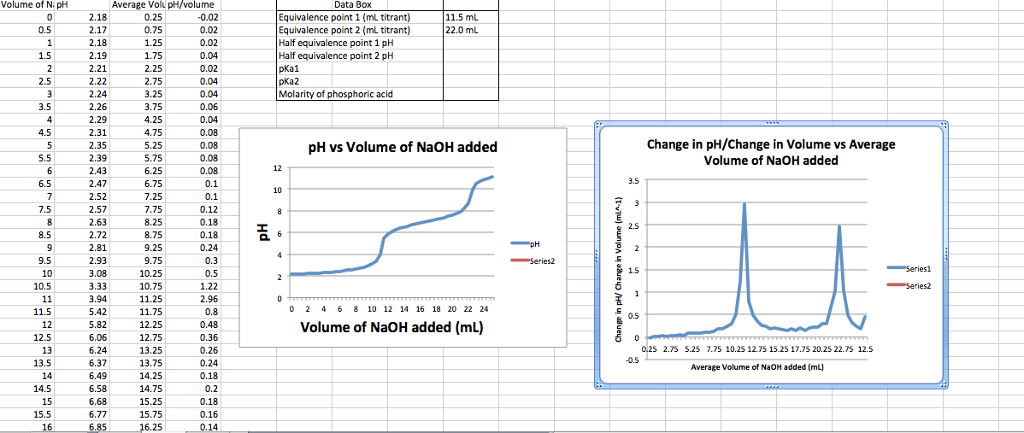
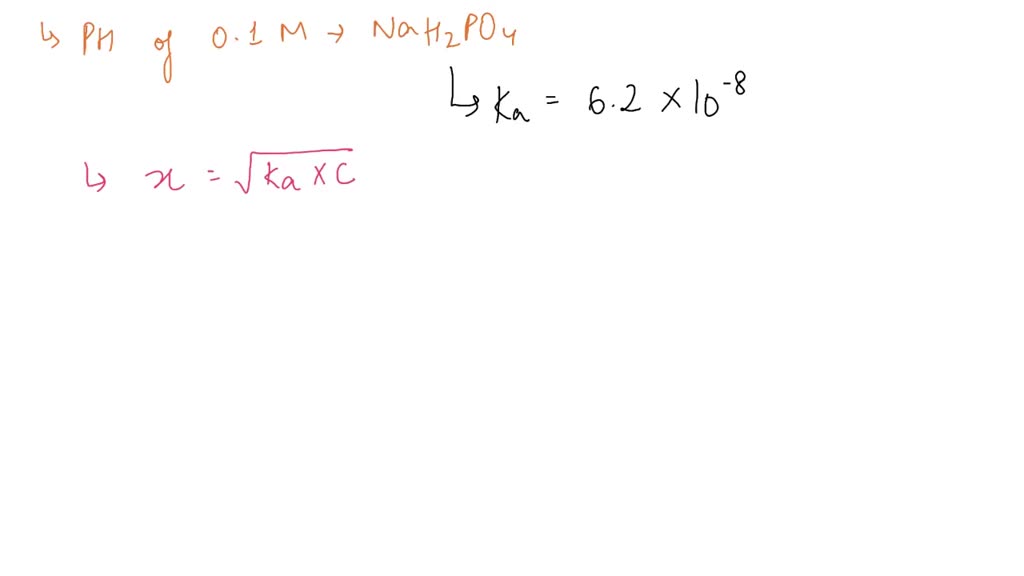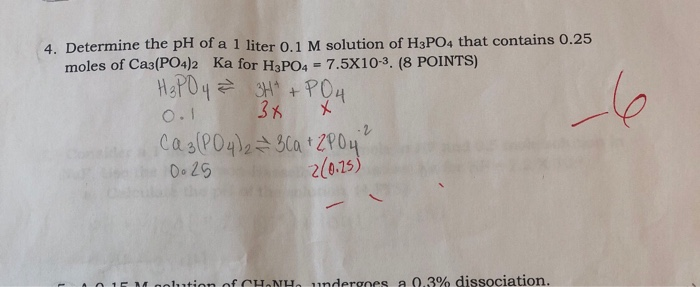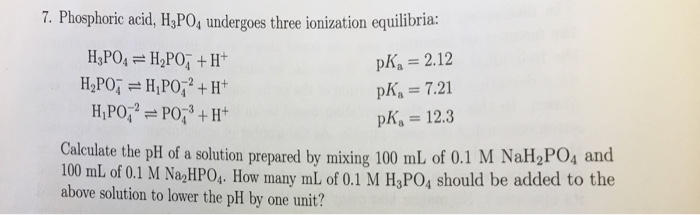Calculate the pH value of buffer solution made by mixing together 100 mL of 0.100 M phosphoric acid (H_3PO_4) and 50 mL of 0.400 NaH_2PO_4. (The K_a of phosphoric acid is 7.08 times 10^{-3}) | Homework.Study.com
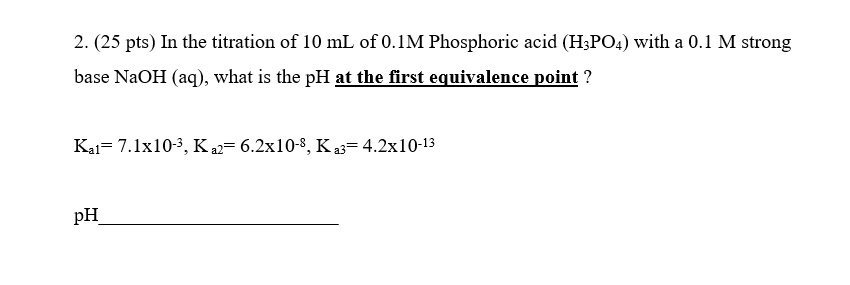
SOLVED: 2. (25 pts) In the titration of 10 mL of 0.1M Phosphoric acid (HzPO4) with a 0.1 M strong base NaOH (aq), what is the pH at thefirst equivalence point ?

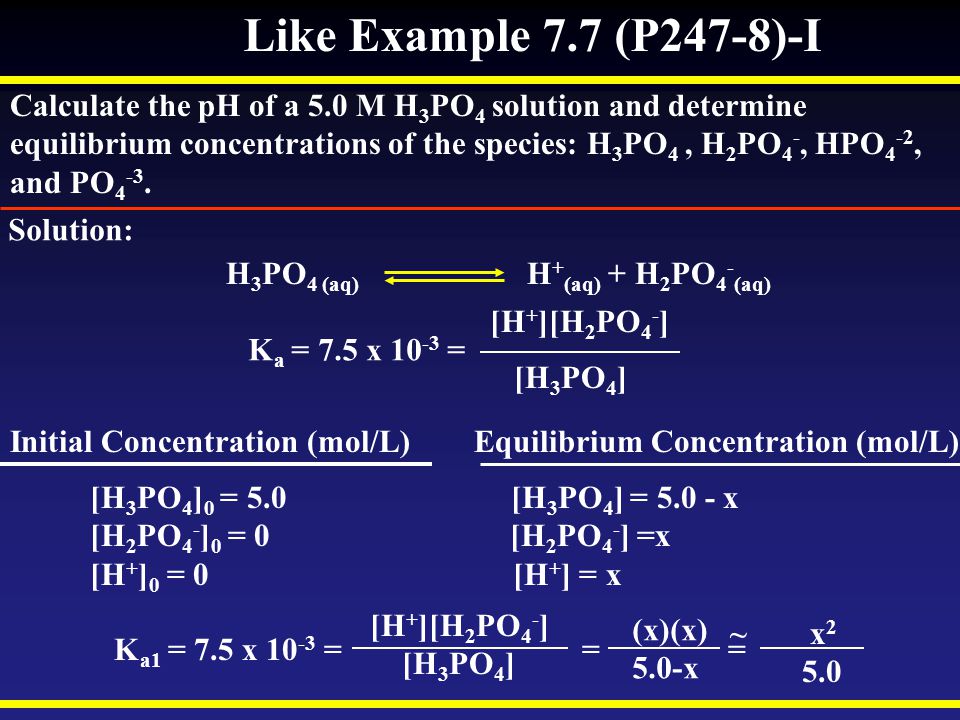
SOLVED: 50 mL of 0.1 M H3PO4 is titrated with 0.1 M NaOH. Phosphoric acid has three pKa values: pKa1 =2.12, pKa2 = 7.21, and pKa3 = 12.3. Please calculate pH when
![pH of 0.1 M H3PO4 acid solution is :[For the given acid: Ka1 = 10^-3, Ka2 = 10^-7 and Ka3 = 10^-12 ] pH of 0.1 M H3PO4 acid solution is :[For the given acid: Ka1 = 10^-3, Ka2 = 10^-7 and Ka3 = 10^-12 ]](https://haygot.s3.amazonaws.com/questions/1844080_1287766_ans_5cdaae94561f4a2599341308914a04c0.jpg)
pH of 0.1 M H3PO4 acid solution is :[For the given acid: Ka1 = 10^-3, Ka2 = 10^-7 and Ka3 = 10^-12 ]
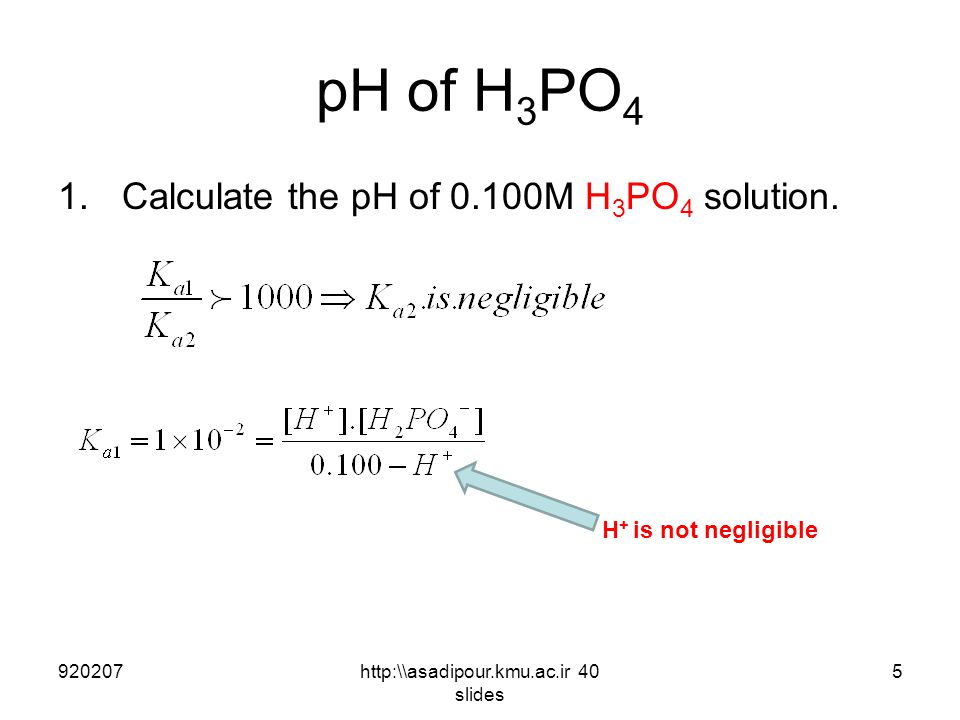
Calculate the concentration of all species of significant concentrations present in 0.1 M H3PO4 solution. - Sarthaks eConnect | Largest Online Education Community
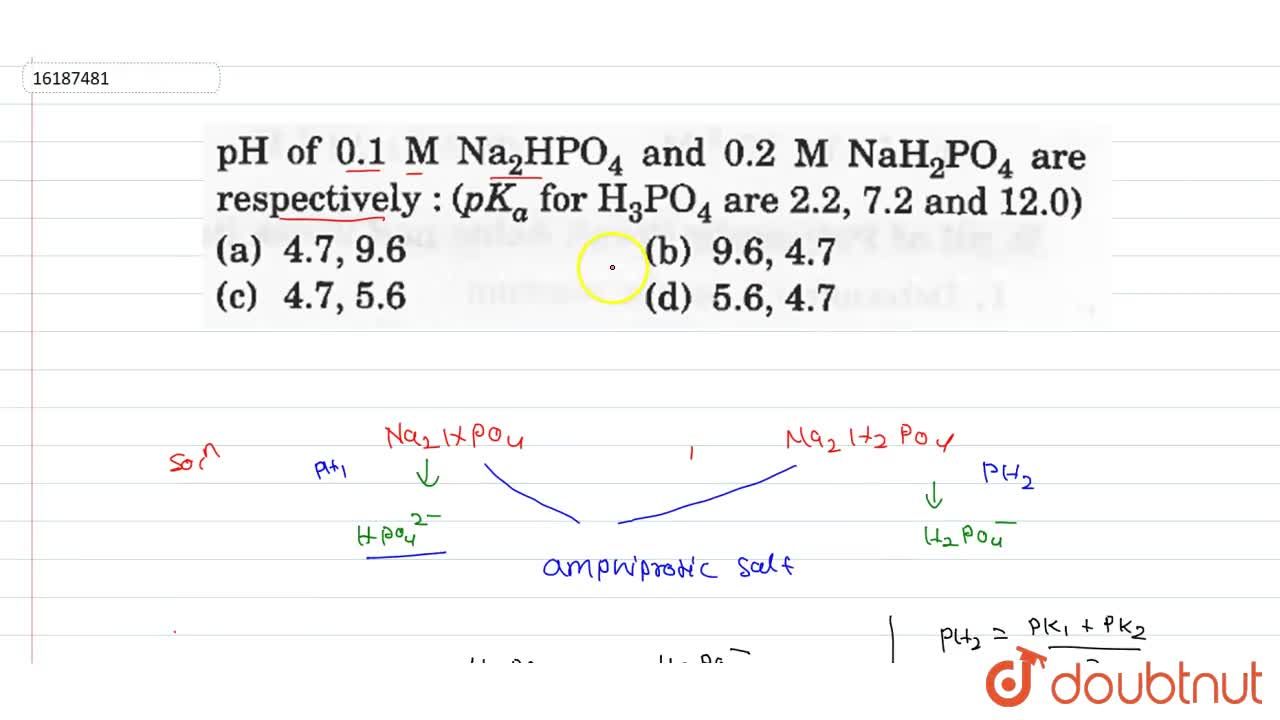
When a large quantity of NaOH added to a buffer solution consisting of H3PO4/NaH2PO4, what will happen to the pH of the solution? - Quora
Calculate the concentration of all species of significant concentrations present in 0.1 M H3PO4 solution. - Sarthaks eConnect | Largest Online Education Community

H2O + H3PO4 H3O^+ + H2PO4^ - ; pK1 = 2.15 H2O + H2PO4^- H3O^+ + HPO4^2 - ; pK2 = 7.20 Hence, pH of 0.01 M NaH2PO4 is:
![In a solution 0.1 M H3PO4 acid, concentration of H^+ is :[Use : Ka1 = 10^-3, Ka2 = 10^-7, Ka3 = 10^-12 ] In a solution 0.1 M H3PO4 acid, concentration of H^+ is :[Use : Ka1 = 10^-3, Ka2 = 10^-7, Ka3 = 10^-12 ]](https://dwes9vv9u0550.cloudfront.net/images/10282364/81604d52-36ea-4174-8f80-9b1dc198cc50.jpg)
In a solution 0.1 M H3PO4 acid, concentration of H^+ is :[Use : Ka1 = 10^-3, Ka2 = 10^-7, Ka3 = 10^-12 ]

OneClass: calculate the PH and the buffer capacity of mixture of 20 ml 1.0 M H3PO4 and 35 ml 0.1 M Na...