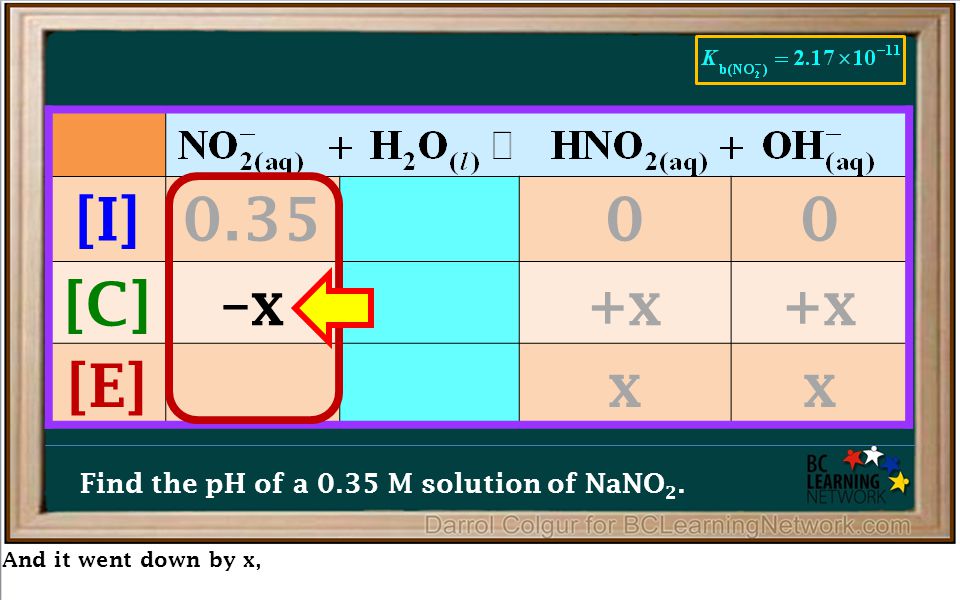
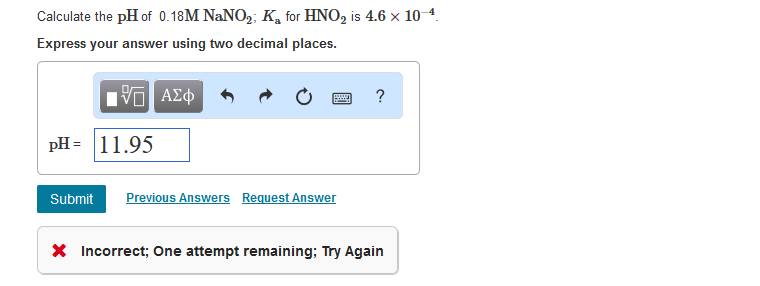
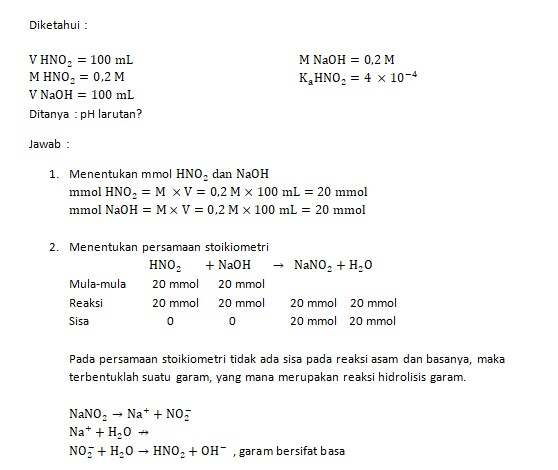
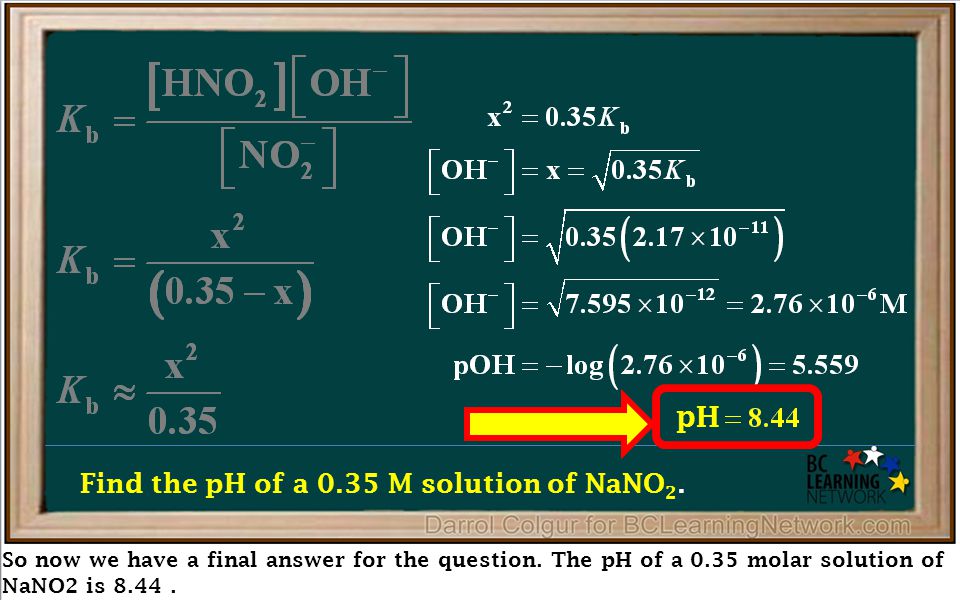
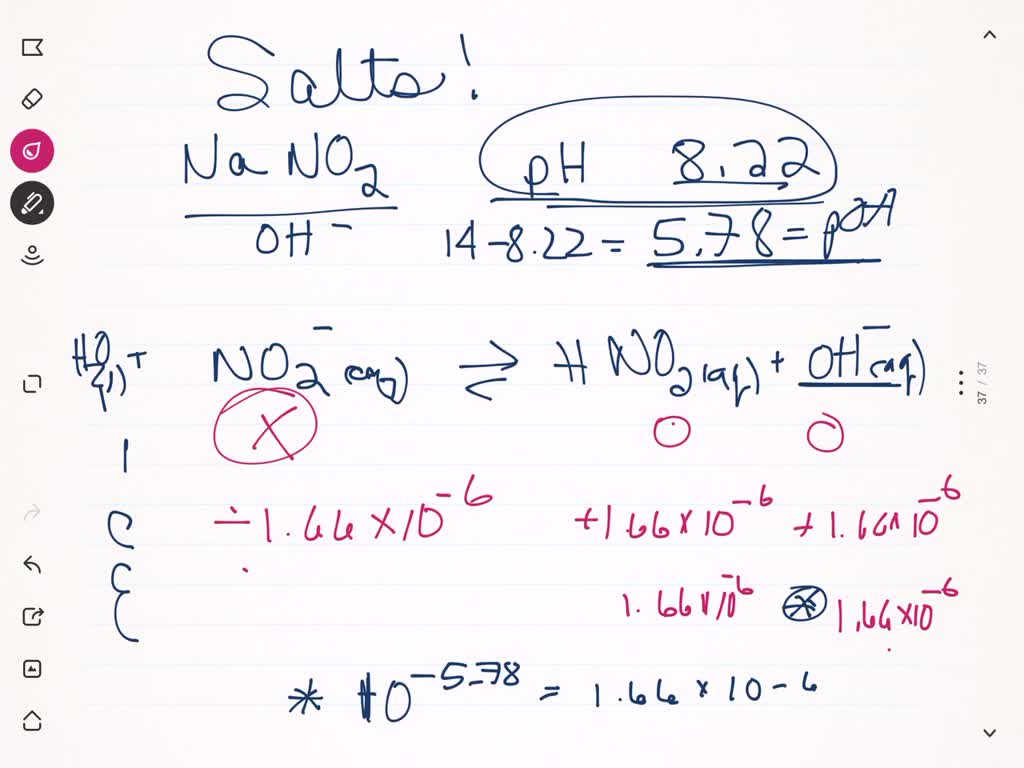OneClass: calculate the pH 1.00L of a buffer that is 0.130 M HNO2 and 0.180 M NaNO2. What is the pH o...

SciELO - Brasil - The Effects of pH Values on Functional Mechanisms of Nitrite Anions for Q235 Carbon Steels in 0.01 mol L<sup>-1</sup> NaNO<sub>2</sub>-HCl Solutions The Effects of pH Values on Functional

50 mL of 2N acetic acid mixed with 10 mL of 1N sodium acetate solution will have an approximate pH of: (Ka = 10^-5) .

SOLVED: What is the pH of a buffer containing 0.225 M NaNO2 and 1M HNO2 . The Ka for the acid is 5.6 x 10-4.

Larutan NaNO2 0,02 M 200ml mempunyai pH sebesar .... (Ka HNO2 = 4,5 . 10^-4) Mohon penjelasannya - Brainly.co.id

The influence of pH on the peak potential and the peak current of NaNO2... | Download Scientific Diagram

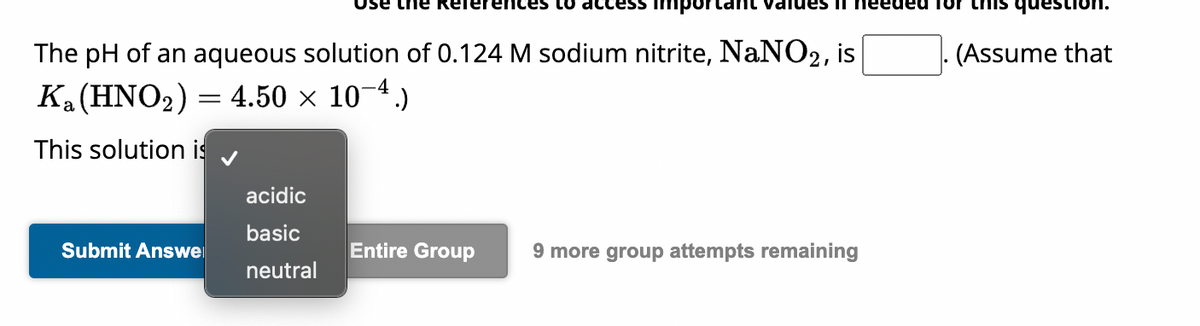
The ionization constant of nitrous acid is 4.5 × 10 4 . Calculate the pH of 0.04 M sodium nitrite solution and also its degree of hydrolysis.

The final major product of the reaction is : Ph - underset(underset(Ph)(|)) overset(overset(OH)(|))C - underset(underset(NH2)(|))CH - CH3 overset(NaNO2 + "HCI") (to)
![What volumes of 0.200 M HNO2 and 0.200 M NaNO2 are required to make 500 mL of a buffer solution with pH =3.00 ? [Ka for HNO2=4.00xx10^(-4)] What volumes of 0.200 M HNO2 and 0.200 M NaNO2 are required to make 500 mL of a buffer solution with pH =3.00 ? [Ka for HNO2=4.00xx10^(-4)]](https://d10lpgp6xz60nq.cloudfront.net/ss/web/437837.jpg)