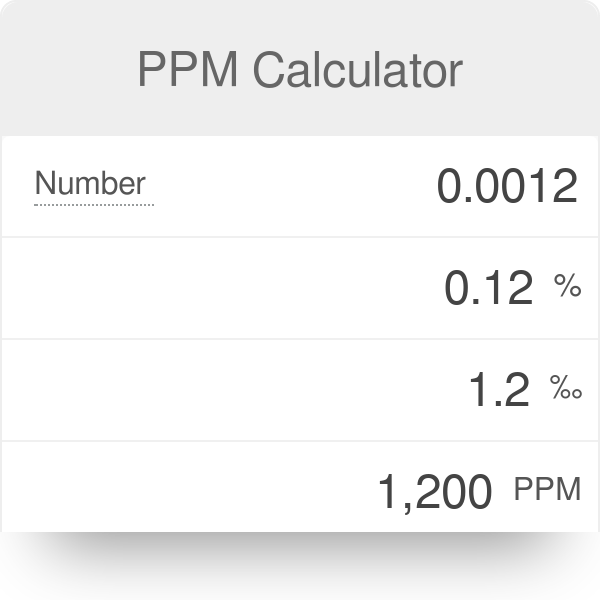
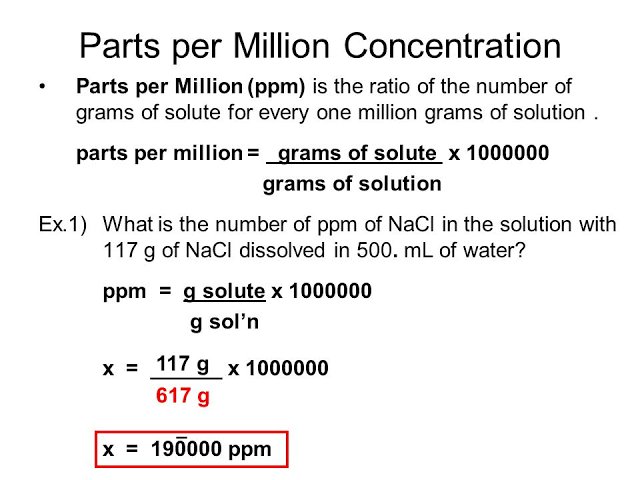
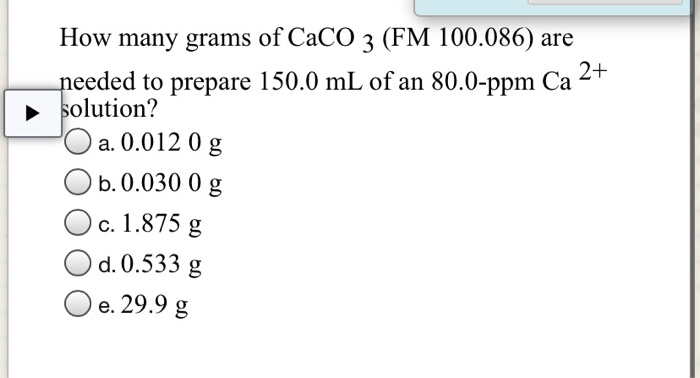
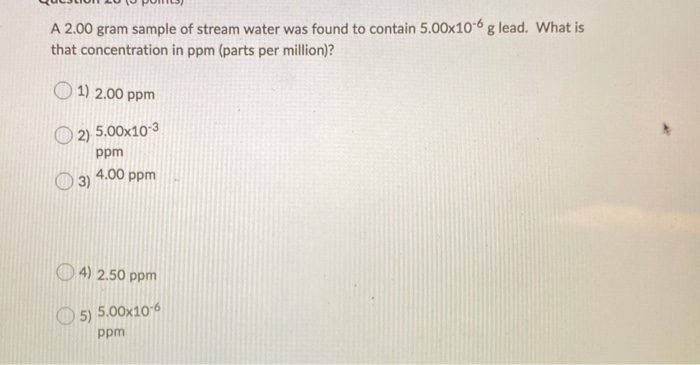
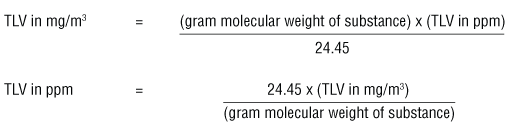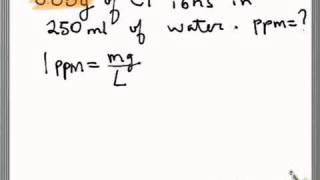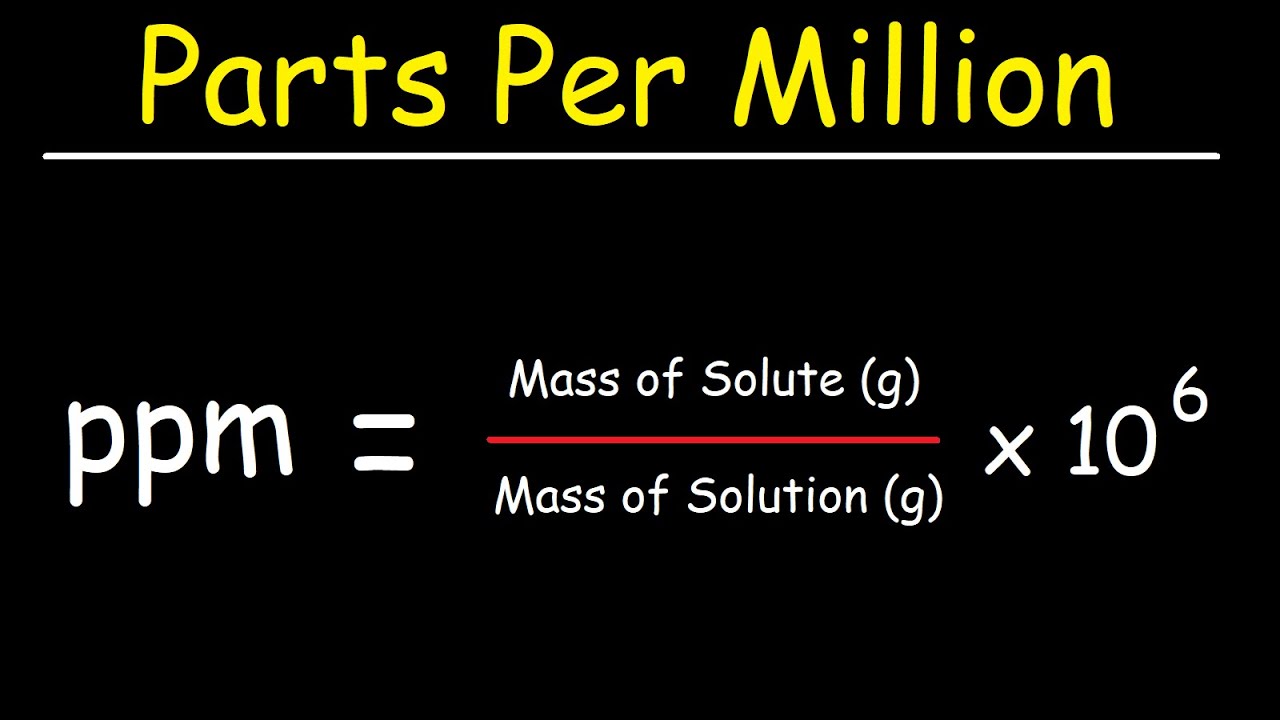PPM (parts per million) PPM = grams solute X 1,000,000 PPM = grams solute X 1,000,000 used when solute is present in very small amounts (The Regents don. - ppt download
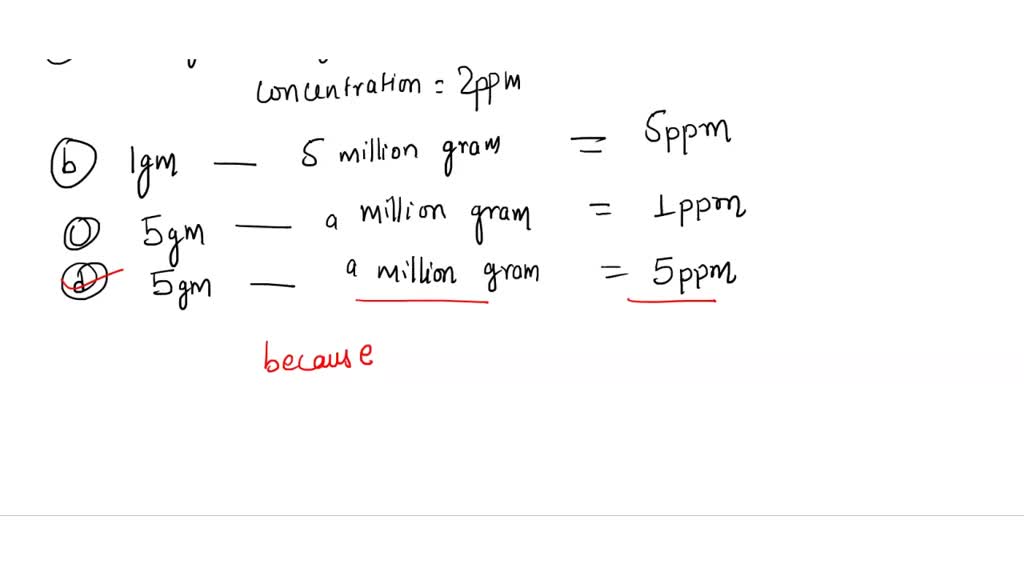
SOLVED: Low concentrations of a substance are usually measured/reported in parts per million (ppm) or parts per billion (ppb). Which one of the following concentrations of a substance is correct? 1 gram

Units of parts per million (ppm) or parts per billion (ppb) are often used to describe the concentrations of solutes in very dilute solutions. The units are defined as the number of

SOLVED: How many grams of mgco3 dissolved per litre gives 84 ppm hardness? A. 70.56 mg/l b. 48.23 mg/l c. 81.49 mg/l d. 66.12 mg/l
_unit-conversion-of-gaseous-pollutant-124-ppm-to-micro-gram-per-cubic-meter-124-ppm-to-g-m-preview-hqdefault.jpg)
Unit conversion of gaseous pollutant | PPM to Micro gram per cubic meter | ppm to µg m³ from ug for microgram Watch Video - HiFiMov.co
1000 g aqueous solution of CaCO3 contains 10 g of calcium carbonate. Concentration of the solution is: (1) 10 ppm (2) 100 ppm (3) 1000 ppm (4) 10000 ppm